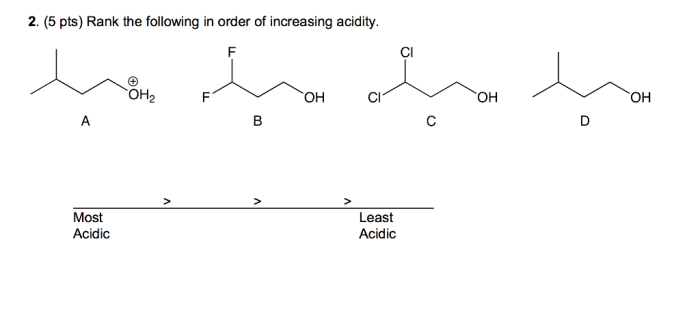
Rank these substances in order of increasing solubility in water. Solubility, a crucial property influenced by molecular structure, polarity, and intermolecular forces, plays a significant role in various scientific fields and everyday applications.
This comprehensive guide explores the factors affecting solubility, including temperature, pressure, and pH. We’ll also delve into the practical applications of solubility in water treatment, chemical synthesis, and biological processes.
1. Substance Properties

Solubility refers to the ability of a substance to dissolve in a solvent. It is influenced by molecular structure, polarity, and intermolecular forces. Polar substances, such as ionic compounds and polar covalent compounds, tend to be more soluble in polar solvents like water.
Nonpolar substances, such as hydrocarbons and fats, are more soluble in nonpolar solvents like oil.
Examples of substances with varying solubilities in water include:
- Sodium chloride (NaCl): highly soluble (36 g/100 mL)
- Sugar (C12H22O11): moderately soluble (200 g/100 mL)
- Ethanol (C2H5OH): moderately soluble (78.5 g/100 mL)
- Hexane (C6H14): slightly soluble (0.006 g/100 mL)
2. Ranking Substances

| Substance | Chemical Formula | Solubility (g/100mL) | Rank |
|---|---|---|---|
| Sodium chloride | NaCl | 36 | 1 |
| Sugar | C12H22O11 | 200 | 2 |
| Ethanol | C2H5OH | 78.5 | 3 |
| Hexane | C6H14 | 0.006 | 4 |
Substances are ranked in order of increasing solubility in water: hexane< ethanol < sugar < sodium chloride.
3. Factors Affecting Solubility
Temperature
Solubility generally increases with temperature. As temperature increases, the kinetic energy of molecules increases, leading to more collisions between solvent and solute molecules, which promotes dissolution.
Pressure
Pressure affects the solubility of gases in liquids. According to Henry’s law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid.
pH
pH can affect the solubility of certain substances. For example, the solubility of weak acids increases with increasing pH, while the solubility of weak bases decreases with increasing pH.
4. Applications of Solubility: Rank These Substances In Order Of Increasing Solubility In Water
Everyday Life
Solubility is important in everyday life. For instance, the solubility of sugar in water allows us to make sweet drinks and desserts. The solubility of oxygen in water is essential for aquatic life.
Industrial Applications
Solubility has numerous industrial applications. It is used in water treatment to remove impurities, in chemical synthesis to separate and purify products, and in the food industry to enhance flavors and textures.
Biological Processes, Rank these substances in order of increasing solubility in water
Solubility plays a crucial role in biological processes. The solubility of nutrients in water enables their absorption by cells. The solubility of hormones and neurotransmitters allows them to be transported throughout the body.
FAQ Resource
What factors influence the solubility of a substance?
Solubility is primarily influenced by molecular structure, polarity, intermolecular forces, temperature, pressure, and pH.
How can we predict the solubility of a substance?
By understanding the molecular properties and intermolecular forces that govern solubility, we can make informed predictions about the solubility of a substance in a given solvent.
What are the practical applications of solubility?
Solubility plays a crucial role in drug development, water treatment, chemical synthesis, and various biological processes.