Unit periodic trends history and the basics – Delving into the realm of unit periodic trends, this exploration embarks on a journey through history and the fundamental principles that govern the periodic table. From its inception to contemporary applications, the study of periodic trends has revolutionized our understanding of element behavior and shaped the course of chemistry.
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number, revealing patterns and trends that govern their properties. These trends, known as periodic trends, provide a powerful tool for predicting and explaining element behavior, enabling scientists to design new materials and solve real-world problems.
1. Periodic Trends
A Historical Overview

Periodic trends refer to the recurring patterns in the properties of elements as we move across and down the periodic table. These trends provide valuable insights into the behavior and characteristics of elements, aiding in their classification and prediction of their properties.
The discovery of periodic trends has a rich history, with many scientists contributing to its development. In the early 19th century, Johann Wolfgang Dobereiner noticed similarities among groups of three elements, known as triads. He observed that the middle element of each triad had properties that were an average of the other two elements.
This observation laid the foundation for the concept of periodicity.
In 1869, Dmitri Mendeleev published his periodic table, which organized elements based on their atomic masses and chemical properties. Mendeleev’s table revealed clear periodic trends, such as the increase in atomic radius down a group and the decrease in ionization energy across a period.
These trends allowed him to predict the properties of undiscovered elements, such as gallium and germanium.
Subsequent research by Henry Moseley in the early 20th century revealed that the atomic number, rather than atomic mass, was the fundamental property governing the periodicity of elements. Moseley’s work further refined the periodic table and provided a more accurate basis for understanding periodic trends.
Periodic trends have played a crucial role in chemistry, enabling scientists to predict and explain the properties of elements and their compounds. They have also guided the development of new materials and technologies, and continue to be an essential tool for understanding the chemical world.
2. Understanding the Basics of Periodic Trends
Periodic trends arise from the underlying electronic structure of atoms. The atomic number, which is the number of protons in the nucleus, determines the number of electrons in the atom. The arrangement of electrons in energy levels, known as electron configuration, influences the chemical properties of elements.
There are several types of periodic trends, each describing a different aspect of an element’s behavior. Some of the most common periodic trends include:
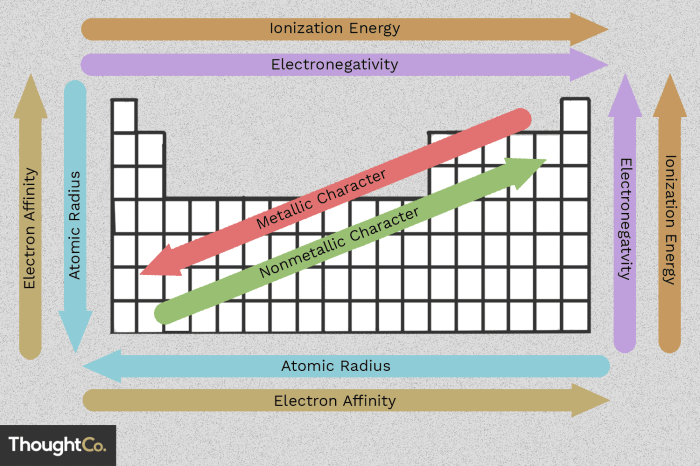
- Atomic radius: The distance from the nucleus to the outermost electron shell. Generally, atomic radius increases down a group and decreases across a period.
- Ionization energy: The energy required to remove an electron from an atom. Ionization energy tends to increase across a period and decrease down a group.
- Electronegativity: The ability of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period and decreases down a group.
The following table illustrates the periodic trend for atomic radius:
| Period | Group 1 | Group 2 | Group 13 | Group 14 | Group 15 | Group 16 | Group 17 | Group 18 |
|---|---|---|---|---|---|---|---|---|
| 1 | H | He | ||||||
| 2 | Li | Be | Ne | |||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar |
| 4 | K | Ca | Ga | Ge | As | Se | Br | Kr |
| 5 | Rb | Sr | In | Sn | Sb | Te | I | Xe |
| 6 | Cs | Ba | Tl | Pb | Bi | Po | At | Rn |
| 7 | Fr | Ra |
3. Applications of Periodic Trends in Chemistry
Periodic trends have wide-ranging applications in chemistry, including:
- Predicting the reactivity of elements: Elements with similar periodic properties tend to exhibit similar chemical behavior. This knowledge can be used to predict the reactivity of an element based on its position in the periodic table.
- Designing and synthesizing new materials: Periodic trends can guide the design and synthesis of new materials with desired properties. For example, understanding the relationship between electronegativity and bond strength can help in designing materials with specific electrical or thermal properties.
- Solving real-world problems: Periodic trends have been applied to solve various real-world problems, such as understanding the behavior of pollutants in the environment, developing new catalysts for industrial processes, and designing drugs with specific biological activities.
4. Exceptions and Limitations of Periodic Trends
While periodic trends provide a valuable framework for understanding the properties of elements, there are certain exceptions and limitations to consider:
- Transition metals: Transition metals exhibit some deviations from the expected periodic trends due to their complex electron configurations and the involvement of d-orbitals.
- Noble gases: Noble gases are highly stable and unreactive, which can lead to deviations from the trends observed for other elements.
- Lanthanides and actinides: Lanthanides and actinides are groups of elements that exhibit unique properties and deviations from the periodic trends due to their large atomic sizes and complex electronic structures.
These exceptions and limitations highlight the need for a nuanced understanding of periodic trends and the factors that can influence the behavior of elements.
FAQs: Unit Periodic Trends History And The Basics
What are periodic trends?
Periodic trends are patterns and regularities in the properties of elements when arranged in the periodic table.
Who discovered periodic trends?
The discovery of periodic trends is attributed to Dmitri Mendeleev, who published his periodic table in 1869.
How can periodic trends be used?
Periodic trends can be used to predict element properties, design new materials, and solve real-world chemistry problems.
Are there exceptions to periodic trends?
Yes, there are some elements that exhibit anomalous behavior and deviate from the expected periodic trends.